Formulation and Characterization of Poly (Ethylene Glycol)-Coated Core-Shell Methionine Magnetic Nanoparticles as a Carrier for Naproxen Delivery: Growth Inhibition of Cancer Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Methionine-Coated Magnetite (Ni1−xCoxFe2O4@Methionine)
2.3. Preparation of PEG-Coated Magnetite (Ni1−xCoxFe2O4@Methionine) NPs
2.4. Characterization
2.5. In Vitro Loading and Release of Naproxen
2.6. In Vitro Cytotoxicity
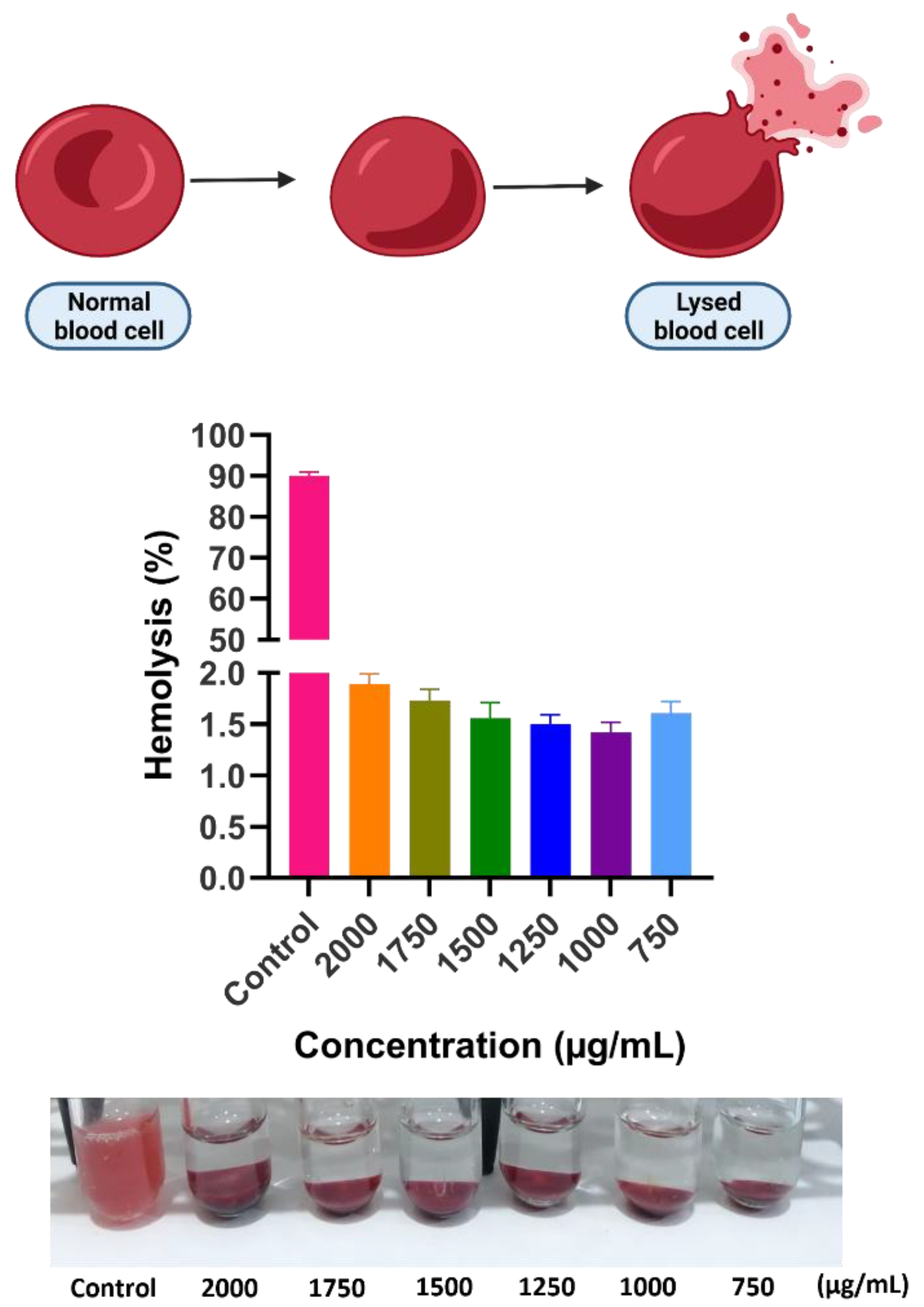
2.7. Hemocompatibility Test
3. Results
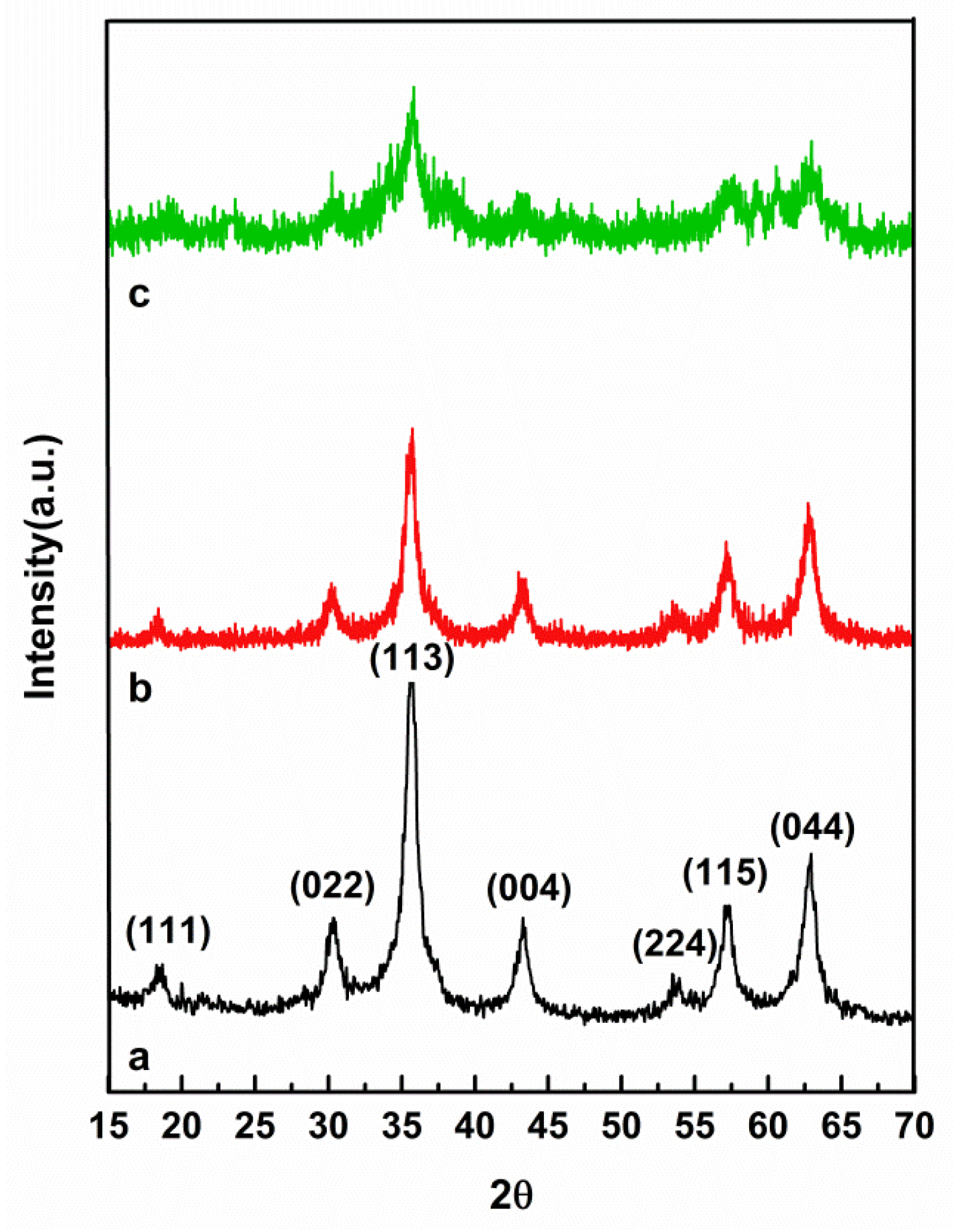
3.1. XRD Analysis
3.2. FTIR Analysis
3.3. Magnetic Properties
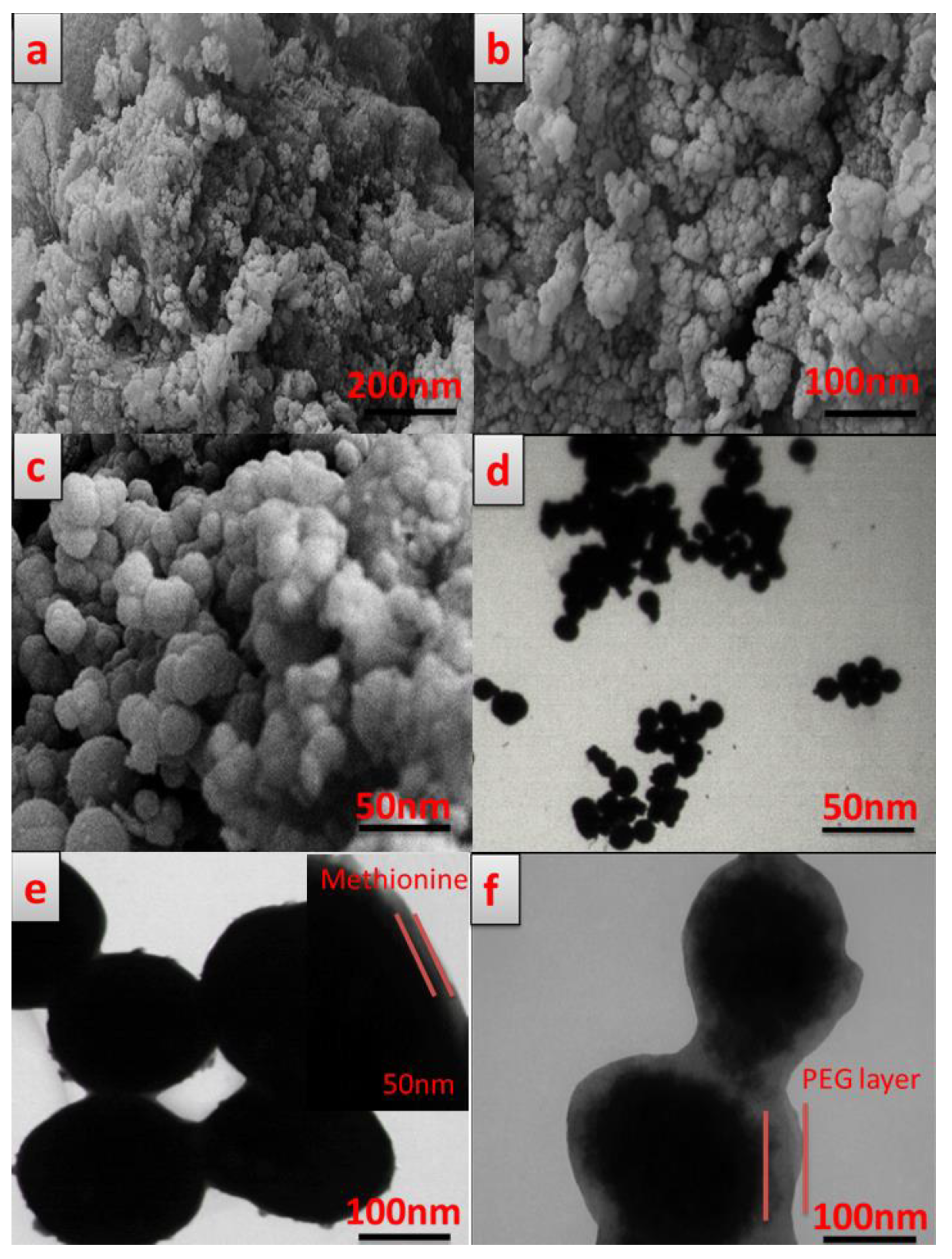
3.4. Morphology Study
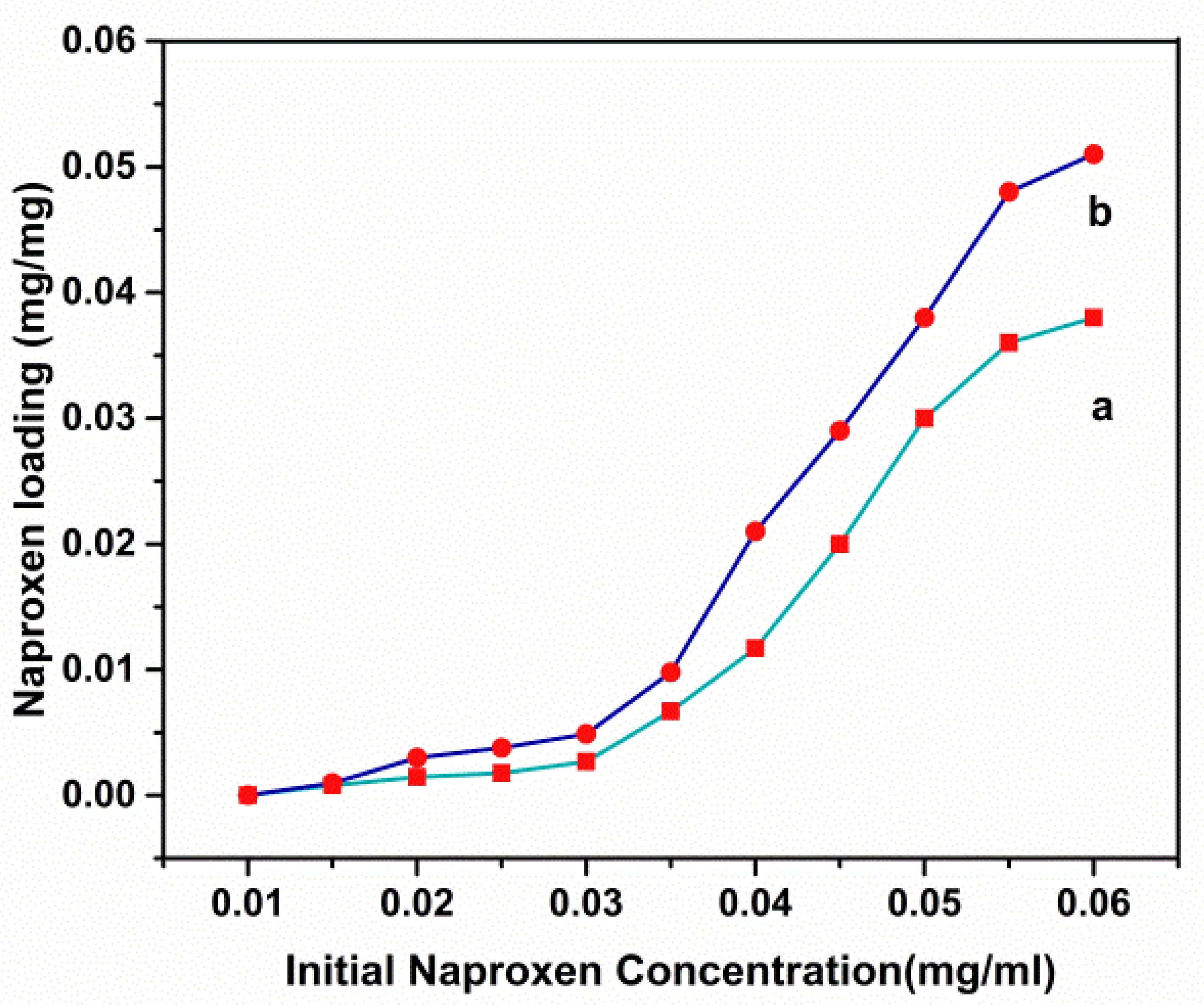
3.5. Drug Loading and Release Behavior In Vitro
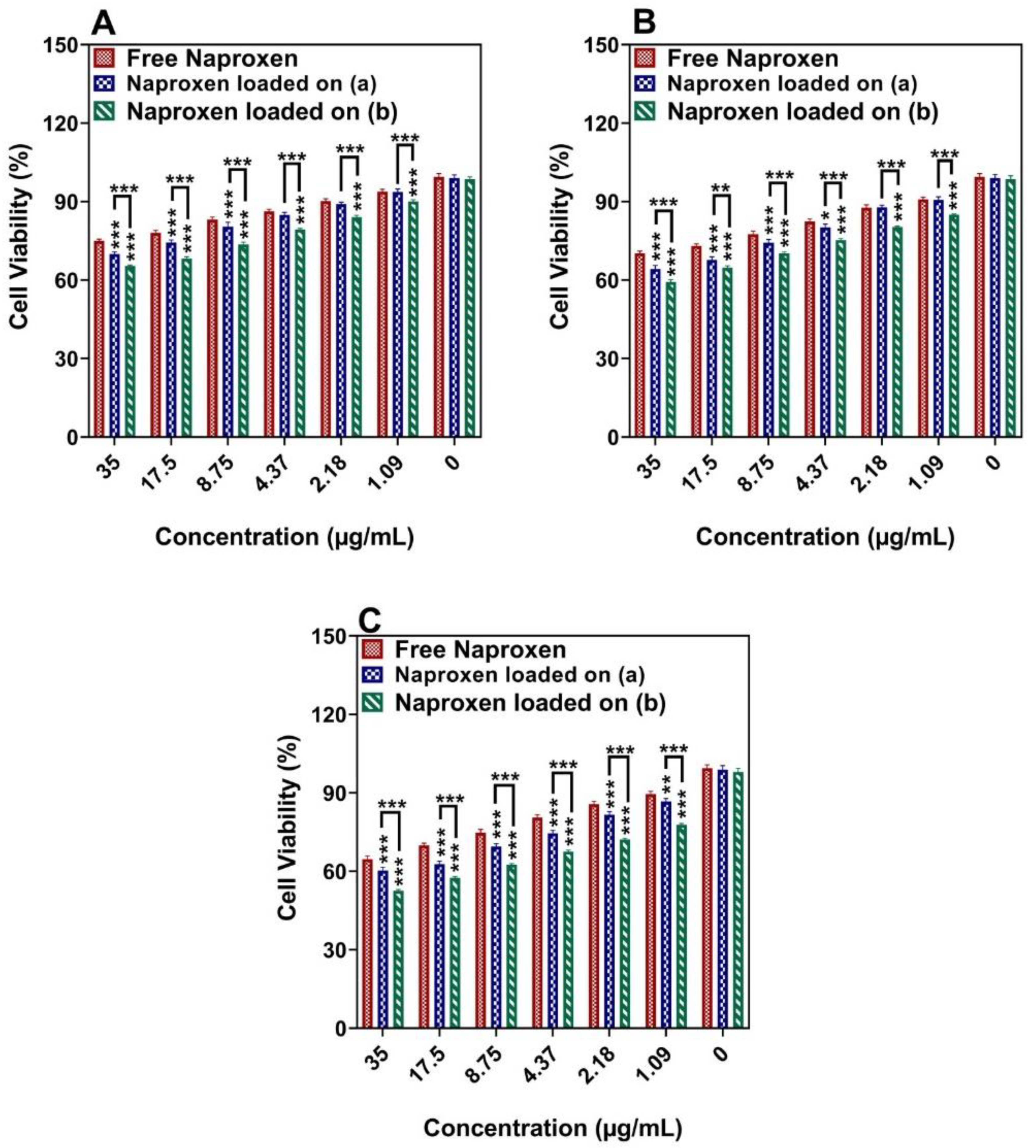
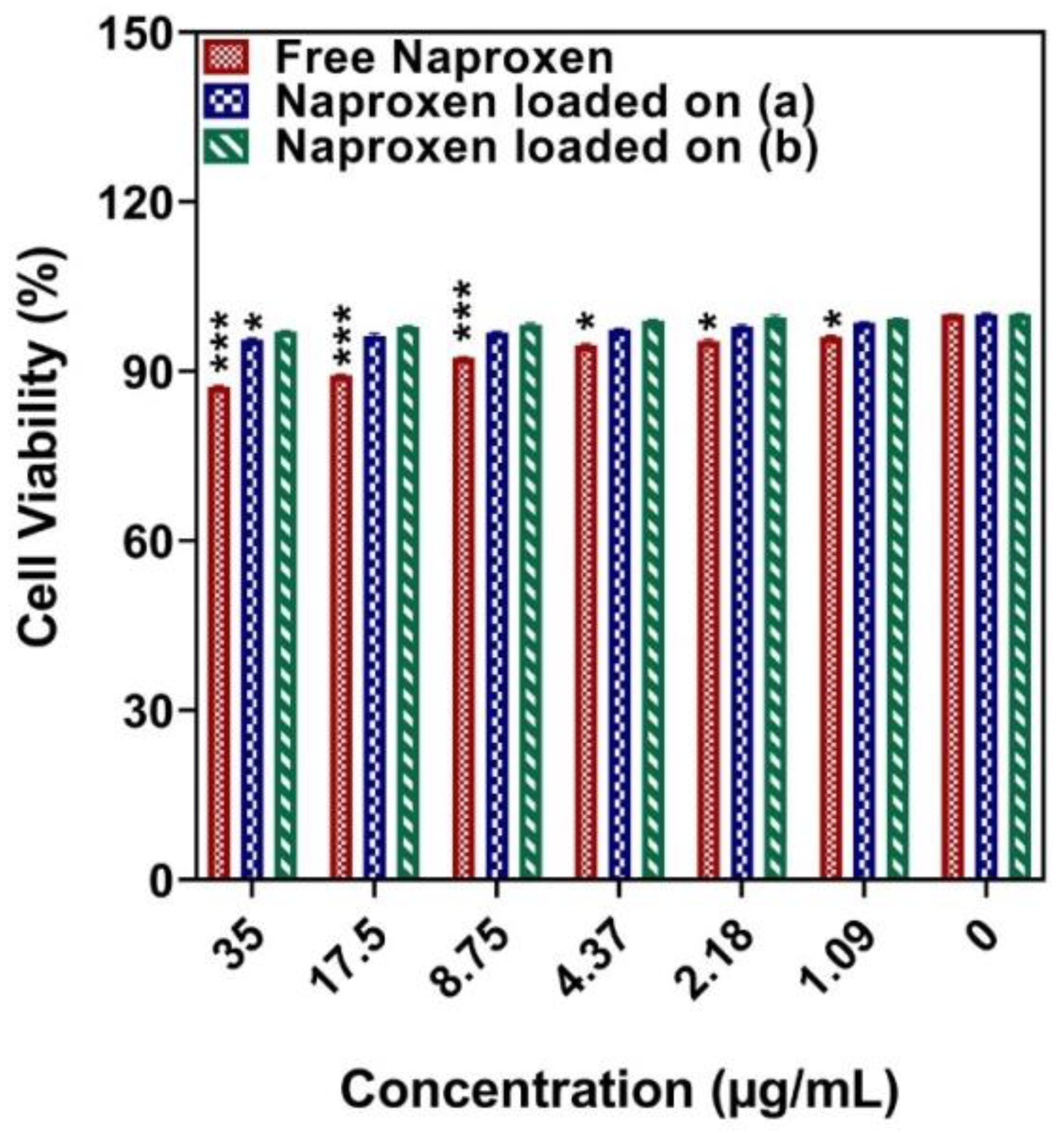
3.6. Cytotoxicity Studies
3.7. Hemocompatibility Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boyle, P.; Levin, B. World Cancer Report 2008; IARC Press: Lyon, France, 2008. [Google Scholar]
- Akbarzadeh, I.; Poor, A.S.; Yaghmaei, S.; Norouzian, D.; Noorbazargan, H.; Saffar, S.; Cohan, R.A.; Bakhshandeh, H. Niosomal delivery of simvastatin to MDA-MB-231 cancer cells. Drug Dev. Ind. Pharm. 2020, 46, 1535–1549. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, I.; Farid, M.; Javidfar, M.; Zabet, N.; Shokoohian, B.; Arki, M.K.; Shpichka, A.; Noorbazargan, H.; Aghdaei, H.A.; Hossein-Khannazer, N.; et al. The Optimized Formulation of Tamoxifen-Loaded Niosomes Efficiently Induced Apoptosis and Cell Cycle Arrest in Breast Cancer Cells. AAPS PharmSciTech 2022, 23, 57. [Google Scholar] [CrossRef] [PubMed]
- Molani, S.; Madadi, M.; Wilkes, W. A partially observable Markov chain framework to estimate overdiagnosis risk in breast cancer screening: Incorporating uncertainty in patients adherence behaviors. Omega 2019, 89, 40–53. [Google Scholar] [CrossRef]
- Sarhadi, M.; Aryan, L.; Zarei, M. The Estrogen Receptor and Breast Cancer: A Complete Review. Trans. Appl. Sci. 2020, 6, 309–314. [Google Scholar]
- Molani, S.; Madadi, M.; Williams, D.L.J.M. Investigating the effectiveness of breast cancer supplemental screening considering radiologists’ bias. MedRxiv 2020. [Google Scholar]
- Singh, S.K.; Singh, S.; Lillard, J.W., Jr.; Singh, R. Drug delivery approaches for breast cancer. Int. J. Nanomed. 2017, 12, 6205. [Google Scholar] [CrossRef] [Green Version]
- Davahli, M.R.; Karwowski, W.; Taiar, R. A System Dynamics Simulation Applied to Healthcare: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 5741. [Google Scholar] [CrossRef]
- Cho, H.-Y.; Lee, Y.-B. Nano-Sized Drug Delivery Systems for Lymphatic Delivery. J. Nanosci. Nanotechnol. 2014, 14, 868–880. [Google Scholar] [CrossRef]
- Akbarzadeh, I.; Fatemizadeh, M.; Heidari, F.; Niri, N.M. Niosomal formulation for co-administration of hydrophobic anticancer drugs into MCF-7 cancer cells. Arch. Adv. Biosci. 2020, 11, 1–9. [Google Scholar]
- Amale, F.R.; Ferdowsian, S.; Hajrasouliha, S.; Kazempoor, R.; Mirzaie, A.; Dakkali, M.S.; Akbarzadeh, I.; Meybodi, S.M.; Mirghafouri, M. Gold nanoparticles loaded into niosomes: A novel approach for enhanced antitumor activity against human ovarian cancer. Adv. Powder Technol. 2021, 32, 4711–4722. [Google Scholar] [CrossRef]
- Khanal, P.; Zargari, F.; Far, B.F.; Kumar, D.; Mogana, R.; Mahdi, Y.K.; Jubair, N.K.; Saraf, S.K.; Bansal, P.; Singh, R.; et al. Integration of System Biology Tools to Investigate Huperzine A as an Anti-Alzheimer Agent. Front. Pharmacol. 2021, 12, 785964. [Google Scholar] [CrossRef] [PubMed]
- Shirzad, M.; Jamehbozorgi, S.; Akbarzadeh, I.; Aghabozorg, H.R.; Amini, A. The Role of Polyethylene Glycol Size in Chemical Spectra, Cytotoxicity, and Release of PEGylated Nanoliposomal Cisplatin. ASSAY Drug Dev. Technol. 2019, 17, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Jamshidifar, E.; Yeganeh, F.E.; Shayan, M.; Yaraki, M.T.; Bourbour, M.; Moammeri, A.; Akbarzadeh, I.; Noorbazargan, H.; Hossein-Khannazer, N. Super Magnetic Niosomal Nanocarrier as a New Approach for Treatment of Breast Cancer: A Case Study on SK-BR-3 and MDA-MB-231 Cell Lines. Int. J. Mol. Sci. 2021, 22, 7948. [Google Scholar] [CrossRef] [PubMed]
- Garg, J.; Chiu, M.N.; Krishnan, S.; Tripathi, L.K.; Pandit, S.; Far, B.F.; Jha, N.K.; Kesari, K.K.; Tripathi, V.; Pandey, S.; et al. Applications of lignin nanoparticles for cancer drug delivery: An update. Mater. Lett. 2021, 311, 131573. [Google Scholar] [CrossRef]
- Roca, A.G.; Carmona, D.; Miguel-Sancho, N.; Bomati-Miguel, O.; Balas, F.; Piquer, C.; Santamaria, J. Surface functionalization for tailoring the aggregation and magnetic behaviour of silica-coated iron oxide nanostructures. Nanotechnology 2012, 23, 155603. [Google Scholar] [CrossRef]
- Yiu, H.H.P. Engineering the multifunctional surface on magnetic nanoparticles for targeted biomedical applications: A chemical approach. Nanomedicine 2011, 6, 1429–1446. [Google Scholar] [CrossRef]
- Cheong, S.; Ferguson, P.; Feindel, K.W.; Hermans, I.F.; Callaghan, P.T.; Meyer, C.; Slocombe, A.; Su, C.-H.; Cheng, F.-Y.; Yeh, C.-S.; et al. Simple Synthesis and Functionalization of Iron Nanoparticles for Magnetic Resonance Imaging. Angew. Chem. Int. Ed. 2011, 50, 4206–4209. [Google Scholar] [CrossRef]
- Cho, N.-H.; Cheong, T.-C.; Min, J.H.; Wu, J.H.; Lee, S.J.; Kim, D.; Yang, J.-S.; Kim, S.; Kim, Y.K.; Seong, S.-Y. A multifunctional core–shell nanoparticle for dendritic cell-based cancer immunotherapy. Nat. Nanotechnol. 2011, 6, 675–682. [Google Scholar] [CrossRef]
- He, X.; Gao, J.; Gambhir, S.S.; Cheng, Z. Near-infrared fluorescent nanoprobes for cancer molecular imaging: Status and challenges. Trends Mol. Med. 2010, 16, 574–583. [Google Scholar] [CrossRef] [Green Version]
- Amiri, S.; Shokrollahi, H. The role of cobalt ferrite magnetic nanoparticles in medical science. Mater. Sci. Eng. C 2013, 33, 1–8. [Google Scholar] [CrossRef]
- Comes, R.; Liu, H.; Khokhlov, M.; Kasica, R.; Lu, J.; Wolf, S.A. Directed Self-Assembly of Epitaxial CoFe2O4–BiFeO3 Multiferroic Nanocomposites. Nano Lett. 2012, 12, 2367–2373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boran, G.; Tavakoli, S.; Dierking, I.; Kamali, A.R.; Ege, D. Synergistic effect of graphene oxide and zoledronic acid for osteoporosis and cancer treatment. Sci. Rep. 2020, 10, 7827. [Google Scholar] [CrossRef] [PubMed]
- Far, B.F.; Asadi, S.; Naimi-Jamal, M.R.; Abdelbasset, W.K.; Shahrivar, A.A. Insights into the interaction of azinphos-methyl with bovine serum albumin: Experimental and molecular docking studies. J. Biomol. Struct. Dyn. 2021, 1–11. [Google Scholar] [CrossRef]
- Sivakumar, M.; Kanagesan, S.; Babu, R.S.; Jesurani, S.; Velmurugan, R.; Thirupathi, C.; Kalaivani, T. Synthesis of CoFe2O4 powder via PVA assisted sol–gel process. J. Mater. Sci. Mater. Electron. 2012, 23, 1045–1049. [Google Scholar] [CrossRef]
- Foroughi, F.; Hassanzadeh-Tabrizi, S.; Bigham, A. In situ microemulsion synthesis of hydroxyapatite-MgFe2O4 nanocomposite as a magnetic drug delivery system. Mater. Sci. Eng. C 2016, 68, 774–779. [Google Scholar] [CrossRef]
- Joshi, H.M.; Lin, Y.P.; Aslam, M.; Prasad, P.V.; Schultz-Sikma, E.A.; Edelman, R.; Meade, T.; Dravid, V.P. Effects of Shape and Size of Cobalt Ferrite Nanostructures on Their MRI Contrast and Thermal Activation. J. Phys. Chem. C 2009, 113, 17761–17767. [Google Scholar] [CrossRef] [Green Version]
- Verde, E.L.; Landi, G.; Gomes, J.; Sousa, M.H.; Bakuzis, A.F. Magnetic hyperthermia investigation of cobalt ferrite nanoparticles: Comparison between experiment, linear response theory, and dynamic hysteresis simulations. J. Appl. Phys. 2012, 111, 123902. [Google Scholar] [CrossRef]
- Fan, H.; Li, B.; Shi, Z.; Zhao, L.; Wang, K.; Qiu, D. A fibrous morphology silica-CoFe2O4 nanocarrier for anticancer drug delivery. Ceram. Int. 2018, 44, 2345–2350. [Google Scholar] [CrossRef]
- Sun, C.; Lee, J.S.H.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef] [Green Version]
- Grange, S.; Chettiar, K.; Arestu, M.; Pilarski, P.; Smitham, P.; Loizidou, M.; Jell, G. Nanotechnology and medical devices: Risk, regulation and ‘meta’ registration. World J. Eng. 2013, 10, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Ghorbani, M.; Hamishehkar, H.; Arsalani, N.; Entezami, A.A. Preparation of thermo and pH-responsive polymer@ Au/Fe3O4 core/shell nanoparticles as a carrier for delivery of anticancer agent. J. Nanopart. Res. 2015, 17, 305. [Google Scholar] [CrossRef]
- Unsoy, G.; Khodadust, R.; Yalcin, S.; Mutlu, P.; Gunduz, U. Synthesis of Doxorubicin loaded magnetic chitosan nanoparticles for pH responsive targeted drug delivery. Eur. J. Pharm. Sci. 2014, 62, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, V.; Menon, J.; Rahimi, M.; Nguyen, K.T.; Wadajkar, A.S. Dual-responsive polymer-coated iron oxide nanoparticles for drug delivery and imaging applications. Int. J. Pharm. 2014, 466, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, H.; Li, L.; Zhou, S.; Liu, Y.-Z. Continuous preparation of Fe3O4 nanoparticles combined with surface modification by L-cysteine and their application in heavy metal adsorption. Ceram. Int. 2016, 42, 4228–4237. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, Y.; Gao, G.; Chen, J.; Hou, R.; Wang, Q.; Liu, L.; Fu, J. Conductive graphene oxide hydrogels reduced and bridged by l-cysteine to support cell adhesion and growth. J. Mater. Chem. B 2017, 5, 511–516. [Google Scholar] [CrossRef]
- Eshrati Yeganeh, F.; Yousefi, M.; Hekmati, M.; Bikhof, M. An experimental research on pH-responsive amino acid-coated Ni(1−x) CoxFe2O4 nanoparticles as a nanocarrier for drug delivery and biological applications. Chem. Pap. 2021, 75, 6047–6057. [Google Scholar] [CrossRef]
- Zarghi, A.; Arfaei, S. Selective COX-2 inhibitors: A review of their structure-activity relationships. Iran. J. Pharm. Res. 2011, 10, 655. [Google Scholar]
- Akbarzadeh, I.; Tabarzad, M.; Khazraei, H.; Ostovan, V. Development of a novel niosomal formulation for Gabapentin. Iran. J. Colorectal Res. 2021, 9, 149–157. [Google Scholar]
- Sahrayi, H.; Hosseini, E.; Karimifard, S.; Khayam, N.; Meybodi, S.M.; Amiri, S.; Bourbour, M.; Far, B.F.; Akbarzadeh, I.; Bhia, M.; et al. Co-Delivery of Letrozole and Cyclophosphamide via Folic Acid-Decorated Nanoniosomes for Breast Cancer Therapy: Synergic Effect, Augmentation of Cytotoxicity, and Apoptosis Gene Expression. Pharmaceuticals 2021, 15, 6. [Google Scholar] [CrossRef]
- Vajedi, F.S.; Dehghani, H.; Zarrabi, A. Design and characterization of a novel pH-sensitive biocompatible and multifunctional nanocarrier for in vitro paclitaxel release. Mater. Sci. Eng. C 2021, 119, 111627. [Google Scholar] [CrossRef]
- Lingaraju, K.; Basavaraj, R.; Jayanna, K.; Bhavana, S.; Devaraja, S.; Swamy, H.K.; Nagaraju, G.; Nagabhushana, H.; Naika, H.R. Biocompatible fabrication of TiO2 nanoparticles: Antimicrobial, anticoagulant, antiplatelet, direct hemolytic and cytotoxicity properties. Inorg. Chem. Commun. 2021, 127, 108505. [Google Scholar] [CrossRef]
- Gong, P.; Wang, Y.; Zhang, P.; Yang, Z.; Deng, W.; Sun, Z.; Yang, M.; Li, X.; Ma, G.; Deng, G.; et al. Immunocyte Membrane-Coated Nanoparticles for Cancer Immunotherapy. Cancers 2020, 13, 77. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, F.; Li, L.; Zheng, Y. Design and development of novel MRI compatible zirconium-ruthenium alloys with ultralow magnetic susceptibility. Sci. Rep. 2016, 6, 24414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yalcin, B.; Ozcelik, S.; Icin, K.; Senturk, K.; Arda, L. Structural, optical, magnetic, photocatalytic activity and related biological effects of CoFe2O4 ferrite nanoparticles. J. Mater. Sci. Mater. Electron. 2021, 32, 13068–13080. [Google Scholar] [CrossRef]
- Panwar, V.; Kumar, P.; Bansal, A.; Ray, S.S.; Jain, S.L. PEGylated magnetic nanoparticles (PEG@Fe3O4) as cost effective alternative for oxidative cyanation of tertiary amines via CH activation. Appl. Catal. A Gen. 2015, 498, 25–31. [Google Scholar] [CrossRef]
- Patnaika, S.; Rao, K.M.; Sai, V. Cytotoxicity Studies on Naproxen and Piroxicam Nanoformulations. J. Nanopart. Res. 2017, 1, 1. [Google Scholar] [CrossRef]
- Bondarenko, O.M.; Ivask, A.; Kahru, A.; Vija, H.; Titma, T.; Visnapuu, M.; Joost, U.; Pudova, K.; Adamberg, S.; Visnapuu, T.; et al. Bacterial polysaccharide levan as stabilizing, non-toxic and functional coating material for microelement-nanoparticles. Carbohydr. Polym. 2016, 136, 710–720. [Google Scholar] [CrossRef]
- Dolara, P. Occurrence, exposure, effects, recommended intake and possible dietary use of selected trace compounds (aluminium, bismuth, cobalt, gold, lithium, nickel, silver). Int. J. Food Sci. Nutr. 2014, 65, 911–924. [Google Scholar] [CrossRef]
- Gari, M.K.; Lemke, P.; Lu, K.H.; Laudadio, E.D.; Henke, A.H.; Green, C.M.; Pho, T.; Hoang, K.N.L.; Murphy, C.J.; Hamers, R.J.; et al. Dynamic aqueous transformations of lithium cobalt oxide nanoparticle induce distinct oxidative stress responses of B. subtilis. Environ. Sci. Nano 2021, 8, 1614–1627. [Google Scholar] [CrossRef]


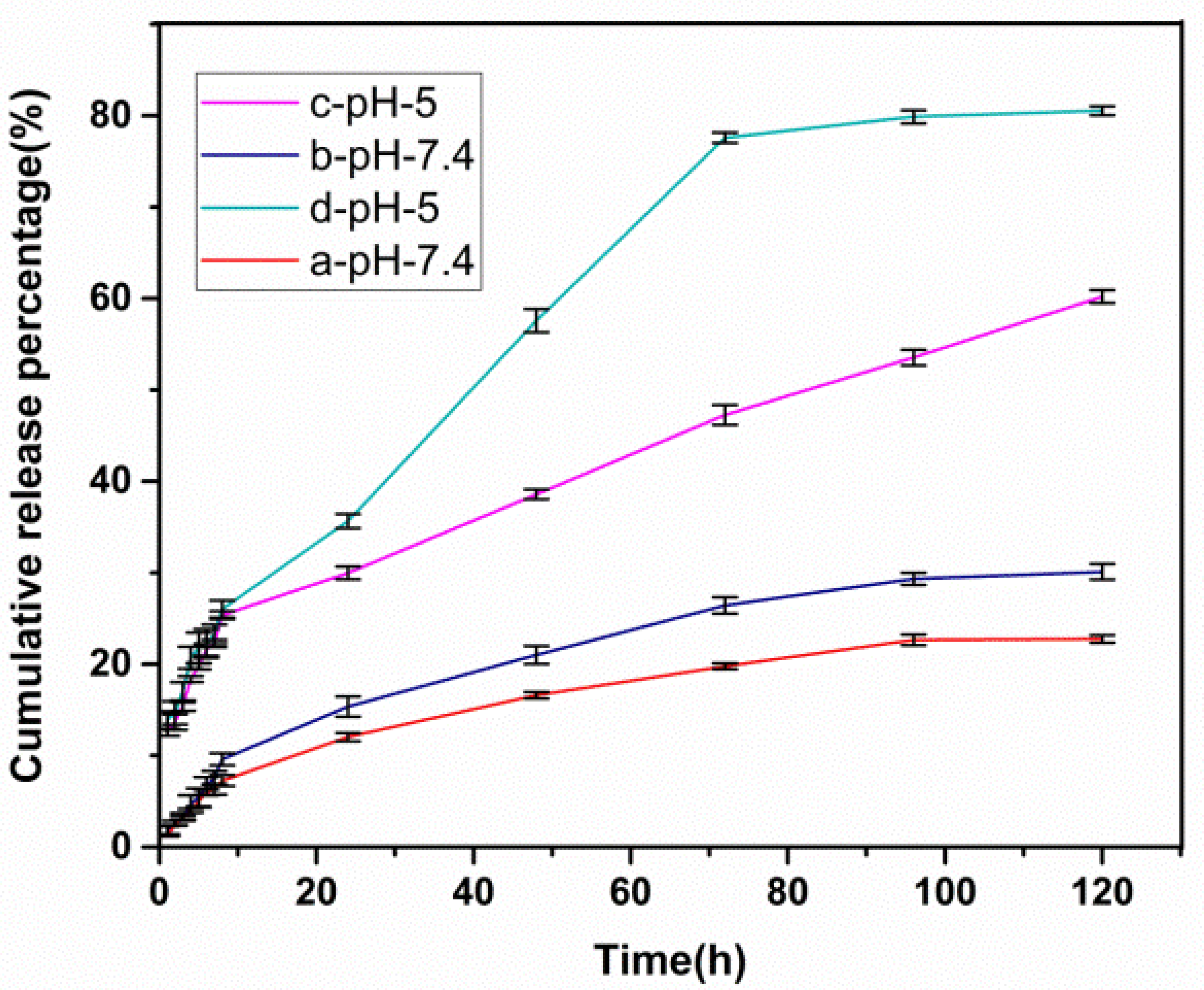

| Release Model | Equation | (a) pH = 5 | (b) pH = 7.4 | (c) pH = 5 | (d) pH = 7.4 |
|---|---|---|---|---|---|
| Zero-Order | Ct = C0 + K0t | R2 = 0.9583 | R2 = 0.9205 | R2 = 0.9363 | R2 = 0.914 |
| First-Order | LogC = LogC0 + Kt/2.303 | R2 = 0.9842 | R2 = 0.9381 | R2 = 0.9607 | R2 = 0.9276 |
| Higuchi | Q = KH√t | R2 = 0.9900 | R2 = 0.9878 | R2 = 0.9794 | R2 = 0.9867 |
| Korsmeyer–Peppas | Mt/M = Ktn | R2 = 0.9852 | R2 = 0.9775 | R2 = 0.9775 | R2 = 0.9689 |
| n = 0.3292 | n = 0.6005 | n = 0.4129 | n = 0.5791 |
| Cell Lines | Incubation Time | IC50 (μg/mL−1 Naproxen) | |
|---|---|---|---|
| Free Naproxen | Naproxen Loaded onto a Naproxen Loaded onto b | ||
| MCF-7 | 24 h | 405.40 ± 97.00 | 117.44 ± 23.27, 138.16 ± 31.45 |
| 48 h | 352.97 ± 65.34 | 114.98 ± 34.26, 66.29 ± 8.71 | |
| 72 h | 186.99 ± 33.83 | 57.45 ± 145.47, 44.03 ± 2.94 | |
| MDA-MB-231 | 24 h | 405.06 ± 144.94 | 138.76 ± 26.93, 176.77 ± 34.64 |
| 48 h | 419.54 ± 295.21 | 98.55 ± 15.45, 92.31 ± 13.72 | |
| 72 h | 218.44 ± 20.18 | 78.81 ± 7.71, 73.79 ± 8.46 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeganeh, F.E.; Yeganeh, A.E.; Yousefi, M.; Farasati Far, B.; Akbarzadeh, I.; Bokov, D.O.; Raahemifar, K.; Soltani, M. Formulation and Characterization of Poly (Ethylene Glycol)-Coated Core-Shell Methionine Magnetic Nanoparticles as a Carrier for Naproxen Delivery: Growth Inhibition of Cancer Cells. Cancers 2022, 14, 1797. https://doi.org/10.3390/cancers14071797
Yeganeh FE, Yeganeh AE, Yousefi M, Farasati Far B, Akbarzadeh I, Bokov DO, Raahemifar K, Soltani M. Formulation and Characterization of Poly (Ethylene Glycol)-Coated Core-Shell Methionine Magnetic Nanoparticles as a Carrier for Naproxen Delivery: Growth Inhibition of Cancer Cells. Cancers. 2022; 14(7):1797. https://doi.org/10.3390/cancers14071797
Chicago/Turabian StyleYeganeh, Faten Eshrati, Amir Eshrati Yeganeh, Mohammad Yousefi, Bahareh Farasati Far, Iman Akbarzadeh, Dmitry Olegovich Bokov, Kaamran Raahemifar, and Madjid Soltani. 2022. "Formulation and Characterization of Poly (Ethylene Glycol)-Coated Core-Shell Methionine Magnetic Nanoparticles as a Carrier for Naproxen Delivery: Growth Inhibition of Cancer Cells" Cancers 14, no. 7: 1797. https://doi.org/10.3390/cancers14071797
APA StyleYeganeh, F. E., Yeganeh, A. E., Yousefi, M., Farasati Far, B., Akbarzadeh, I., Bokov, D. O., Raahemifar, K., & Soltani, M. (2022). Formulation and Characterization of Poly (Ethylene Glycol)-Coated Core-Shell Methionine Magnetic Nanoparticles as a Carrier for Naproxen Delivery: Growth Inhibition of Cancer Cells. Cancers, 14(7), 1797. https://doi.org/10.3390/cancers14071797








